Abstract
"Perhaps it is time to be thinking of a 'vascular immunology' focus in the study of neurological disease, an interesting marriage between cardiovascular and neurodegenerative research."
Introduction
At a workshop in Bologna (Italy) in 2009, Professor Paolo Zamboni openly discussed research that he had been pursuing for the previous few years regarding the role of venous flow abnormalities in multiple sclerosis (MS). His data demonstrated that there were major extracranial venous abnormalities in MS patients. He did not claim that these were 'the' etiology of MS, rather he noted that they were associated with MS and not generally with normal individuals. He referred to the resulting condition as chronic cerebrospinal venous insufficiency, or chronic cerebrospinal venous insufficiency.[1] In this brief article, we would like to bring to bear some of the previous research into the role of the venous vasculature in both MS and other diseases, which may shed some light on the importance of the venous system in neurological diseases.
Historical Considerations
According to Putnam,[2] who discussed vascular abnormalities in MS in the 1930s, the first observations related to abnormal vasculature or effects related to vasculature appeared in the work of Cruveilhier in 1839, more than 170 years ago.[3] In 1863, Rindfleisch noted an engorged vessel in the center of a plaque,[4] and in the same year, Charcot described vascular obstruction in MS.[5] These observations would be noted many times over the next 135 years. Putnam himself appeared convinced that the etiology of MS lay in the venous system. To test his hypothesis, he proceeded to study the effects of obstructed venous flow in the cerebral veins of dogs. These animals developed a number of abnormalities (lesions), many of them similar to encephalitis or MS. He noted at the end of his article: "The similarity between such lesions and many of those seen in cases of multiple sclerosis in man is so striking that the conclusion appears almost inevitable that venular obstruction is the essential immediate antecedent to the formation of typical sclerotic plaques".[6]
The story continues with a reference to Borst, who suggests that vascular obstruction occurs to the point of complete obliteration and hyaline transformation.[7] A similar loss of the venous vasculature has been reported using susceptibility-weighted imaging by Ge et al. in 2009.[8] Some researchers describe the combination of congestion, perivascular hemorrhage and pigments (possibly hemosiderin or iron-related[2,9]) in encephalitis following measles.[10] In the 1980s, Adams found the presence of hemosiderin in the form of old hemorrhage in 30% of cadaver brain MS lesions.[11] More recently, a number of articles on measuring iron using MRI also found 30% or more of MS lesions appeared to have increased iron content.[12–14] Could this hemosiderin correspond, for example, to cases where we see iron deposition with susceptibility-weighted imaging in brain lesions [12]? In another article, iron was shown to build up backward along the venous system in the basal ganglia in the thalamostriate region.[15]
In a recent review of the role of venous reflux, Simka stated: "It is hypothesized that pathological refluxing venous flow in the cerebral and spinal veins increases the expression of adhesion molecules, particularly ICAM-1, by the cerebrovascular endothelium".[16] Along these lines, Bergan demonstrated, by occluding a major vein in the rat, that the number of leukocytes migrating across the vessel wall increased progressively during occlusion.[17–19] Multiple microhemorrhages occurred upstream of the occlusion (usually 20–30 µm in diameter, but some as large as 200 µm). "The venular occlusion experiments showed that reduced flow can rapidly set in motion an inflammatory cascade, including hallmarks like leukocyte adhesion to the endothelium, migration into the interstitium, free radical production and parenchymal cell death that begins soon after occlusion…" Bergan goes on to say: "Elevated pressures can also cause the formation of transcellular gaps through endothelial cells, which may be related to the development of microhemorrhages."
In the 1980s, Schelling believed that there was a significant mechanical nature related to the fact that the vascular damage follows a path opposed to the flow.[20] He quotes Carswell as saying: "In inflammation, the local congestion commences in the capillaries, afterwards extends to the small veins, but never to large branches; in mechanical congestion (by venous flow inversion) the blood accumulates first in the venous trunks, which are always conspicuous, and afterwards in the branches and capillaries.".[21] Further evidence of this mechanical effect comes from observations of Allen, who noticed the widened vascular beds around veins and the central widening of the venous tree indicative of intermittent increases in cerebral pressure.[22] It is also worth looking into Fog's work. He summarizes his results from a series of cadaver brain studies as "thirty plaques showed that they definitely followed the course of the veins, so that course and dimensions of the veins determine the shape, course and dimension of the plaques.".[23] He also closes his work with the comment: "Consequently, multiple sclerosis, pathologico-anatomically, must be considered a periphlebitis, as proved by the author in 1948 in the case of plaques of the spinal cord.".[24]
In summary, during the last two centuries there has been ample evidence that the venous system is strongly associated with MS. Integrating this information together yields a more complete picture of the role the veins play, from mechanical issues to immunological issues. As discussed later, this will be complemented by information regarding perfusion deficits, evidence for venous effects in other diseases and the clinical evidence for major venous insufficiency in MS seen with modern imaging technologies today.
Perfusion Deficits in Multiple Sclerosis
Recently, it has been demonstrated that there is reduced perfusion and even loss of small medullary vein visibility in MS.[8] Juurlink discusses the role of hypoperfusion in MS.[25] He comments that the reduced perfusion can be detrimental to oligodendrocytes, preferentially affects white matter, causes demyelination and leads to microglial activity. He notes that these can be most marked in the optic nerve and tract. He then states: "There is now ample evidence that ischemic insults of sufficient severity can cause upregulation of cell adhesion molecules onto the endothelial cells, thus allowing infiltration of leukocytes into the brain parenchyma, resulting in an inflammatory lesion." He goes on to point out that hypertension of genetically susceptible lesions leads to vascular damage, which in turn leads to ischemia. There is actually a body of evidence suggesting reduced perfusion in MS patients. Back in the 1980s, Swank et al. found that past the age of 40 years, MS patients had markedly reduced blood flow compared with normal individuals.[26] Using MRI, there has been a thrust in the last 10 years to study perfusion in MS. The work of Meng Law[27] and others[28–32] demonstrates that there is reduced perfusion as a function of severity of disease. Law et al. reported a significant decrease of cerebral blood flow and a prolongation of mean transit time in the normal-appearing white matter (NAWM) at the level of the lateral ventricles in MS patients.[27] These reductions often appear in the basal ganglia and thalamus and have been related to fatigue.[30,31,33] A study of seven patients with relapsing–remitting MS revealed decreased relative cerebral blood volume (CBV) in chronic lesions and further reduced relative CBV in one acute lesion in white matter compared with that in gray matter.[27] Haselhorst et al. examined 25 patients with MS and found that acute lesions had significantly higher relative CBV than NAWM and that chronic plaques had significantly lower relative CBV than NAWM.[34] Inflammatory activity can cause compensative vasodilatation and result in increased cerebral blood flow and CBV, which is found in enhancing lesions. On the other hand, any evidence of increased perfusion in some chronic nonenhancing lesions can be explained by lesion reactivity with new vascular inflammatory changes.
Clinical Evidence of Venous Abnormalities
Optical neuritis is another common condition for MS patients. Evaluating periphlebitis retinae involves detecting sheathing and hemorrhage in veins in the retina. Lightman et al. demonstrated that 3.5 years after initial onset of acute idiopathic optic neuritis, eight out of 14 patients who had vascular abnormalities in a first episode of optic neuritis went on to develop MS, while only five out of 32 patients without vascular abnormalities went on to develop MS.[35] The overall incidence of patients with optic neuritis going on to be diagnosed as clinically definite was 13 out of 46 subjects, or 28%. The authors go on to draw the following conclusion: "We therefore suggest that the sheathing of retinal vessels that we observed opthalmoscopically is the visible clinical sign of the perivascular lymphocytic infiltration and accompanying oedema which characterizes the lesions of MS."
Venous abnormalities might best be viewed from an embryological background when trying to understand the presence of truncular venous malformations.[36] The development of the venous system begins in the early stages of the fetus and has a well-known developmental etiology. An example of abnormal venous development occurs in the case of primary Budd–Chiari syndrome, which can lead to severe portal hypertension. For jugular veins, development occurs as the neck lengthens and its drainage level shifts toward the cephalad part of the precardinal vein, which later develops into the internal jugular vein.
There is a very timely review of the importance of jugular venous reflux.[37] In this article, the authors note that without a competent jugular valve, and with prolonged venous reflux, the individual may develop venous hypertension or occlusion.[38] Several disorders are now associated with internal jugular vein incompetence, including: transient global ischemia;[39] transient blindness;[40] cough headache;[41] and primary exertional headache.[42] Many other conditions can also generate some level of this elevated venous pressure, such as congestive heart disease, tricuspid valve regurgitation, primary pulmonary hypertension and chronic obstructive pulmonary disease. Valves can also tend to breakdown with age. In veins without valves, simply reversing the pressure gradient can produce reflux. This condition "might impede cerebral venous outflow and induce neurologic dysfunction," according to Chung and Hu.[37] These conditions can occur during Valsalva-like activities, such as coughing, heavy lifting and other strenuous activities.
Of significance to MS patients currently undergoing treatment with balloon angioplasty are studies of idiopathic intracranial hypertension. In a group of MS patients, it has been demonstrated using 3D magnetic resonance venography that in the order of 90% of these patients have sinovenous stenoses.[43] There are a variety of different methods used to image these abnormalities (including computer tomography angiography), but magnetic resonance venography appears to be the safest and provides sufficient information to make the diagnosis.[44]
Zamboni et al. assessed the venous system using transcranial color-coded duplex sonography.[45] They comment that venous reflux overloads the microcirculation, leading to the activation of MMPs that in turn digest basement membrane-type IV collagen and fibronectin, allowing migration of cells and proteins into the CNS. Venous reflux also facilitates erythrocyte diapedesis, leading to perivenous iron deposits. Pericapillary fibrin cuff and vessel thickening are markers of venous insufficiency. "In our study, the involvement of the veins of the gray matter was significantly associated with the worse disability scores of our MS population."
Ludyga et al. report on their first 330 cases.[46] Apart from minor problems with difficulty removing the angioplastic balloon, occlusion of stent or local bleeding from the groin, there were no major complications (severe bleeding, venous thrombosis, stent migration or injury to the nerves) related to the procedure. They reported that approximately 60% had bilateral stenoses of a jugular vein and 40% had unilateral stenoses. Balloon angioplasty alone was performed in approximately 55% of cases, whereas the stenting of at least one vein was required in approximately 45%. Surgical interventions were planned in 330 patients. In 11 cases (3%), no obvious pathology was found, despite signs of chronic cerebrospinal venous insufficiency on color Doppler sonography and/or MR venography.
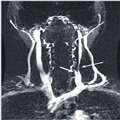
Zamboni thought outside the box by looking for extracranial vascular abnormalities to understand MS. His focus was using ultrasound as a biomarker for stenoses and flow changes. His gold standard was the angiographic scanning carried out during treatment. Our own work in this area has focused on using MR angiographic methods to collect time-resolved data post-contrast to image the arterial and venous phases. With appropriate post-processing, we can then separate 3D images of both arteries and veins. Figure 1 shows an example of an MR venogram revealing a tight stenosis in the left internal jugular vein. This is the type of venous malformation that would have served as a candidate for percutaneous transluminal angioplasty in their study. Currently, there are a number of ongoing studies around the world set in motion to try and validate the findings of Zamboni et al. and quantify both the frequency of stenoses and the flow abnormalities in both MS patients and in normal volunteers.
|
Figure 1. A magnetic resonance venogram from a multiple sclerosis patient showing a tight stenosis (long, thin arrow) of the left internal jugular vein in the region of the confluence of a vertebral vein (short, thick arrow) into the trunk of the left internal jugular vein.
|
Conclusion
The above discussions provide varied evidence that the venous system plays a major role, or in the least is associated with, MS. Past efforts in immunology have been impressive. More recent efforts in understanding how vascular abnormalities lead to immunological effects are suggestive. Perhaps it is time to be thinking of a 'vascular immunology' focus in the study of neurological disease, an interesting marriage between cardiovascular and neurodegenerative research. A recent review has promoted this concept as it relates to vascular endothelial health[47] and is written focusing on MS as a vascular disease. It behooves us to follow this advice, especially in light of the work of Zamboni and now many others following in his footsteps worldwide that suggest the venous vascular system may be strongly associated with MS.
http://www.medscape.com/viewarticle/734517_5